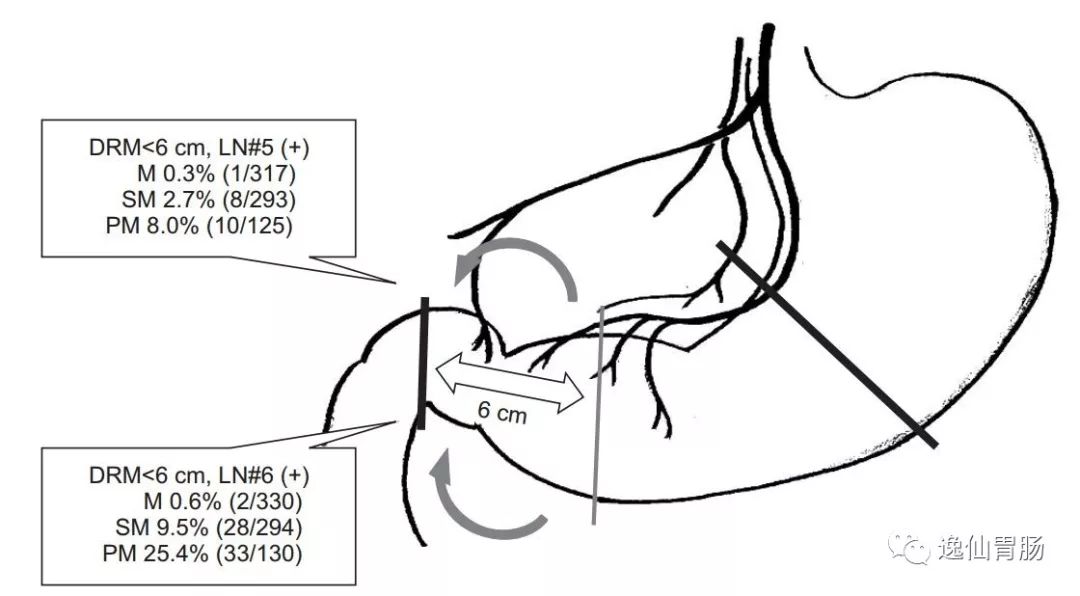
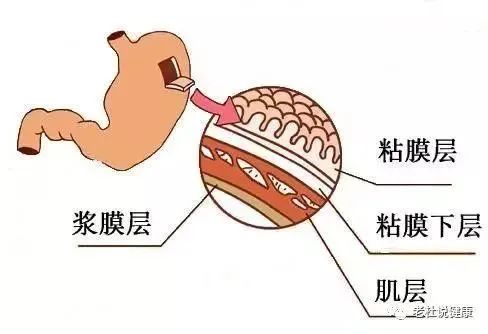
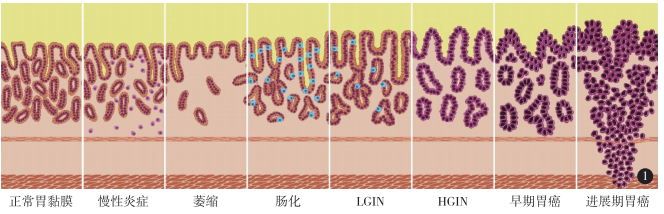
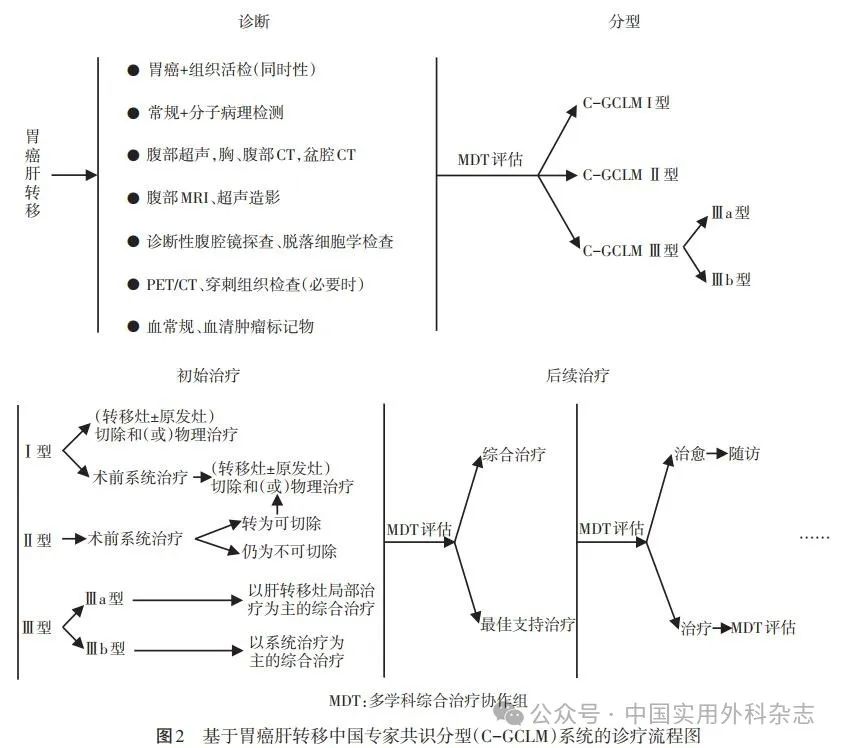
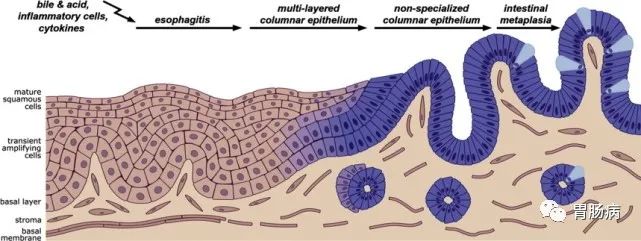
参考文献4a3帝国网站管理系统
(在框内滑动手指即可浏览)4a3帝国网站管理系统
4a3帝国网站管理系统
[1] Sung H,Ferlay J,Siegel RL,et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin,2021,71(3): 209-249. 4a3帝国网站管理系统
[2] Xia C,Dong X,Li H,et al. Cancer statistics in China and United States,2022: profiles,trends,and determinants[J]. Chin Med J(Engl),2022,135(5): 584-590. 4a3帝国网站管理系统
[3] Cao W,Chen HD,Yu YW,et al. Changing profiles of cancer burden worldwide and in China: A secondary analysis of the global cancer statistics 2020[J]. Chin Med J(Engl),2021,134(7): 783-791.4a3帝国网站管理系统
[4] Zeng H,Chen W,Zheng R,et al. Changing cancer survival in China during 2003-15: A pooled analysis of 17 population-based cancer registries[J]. Lancet Glob Health,2018,6(5): e555-e567.4a3帝国网站管理系统
[5] 闫超,陕飞,李子禹. 2020年全球胃癌负担分析: 聚焦中国流行现状[J]. 中国肿瘤,2023,32(3): 161-170. 4a3帝国网站管理系统
[6] Ju W,Zheng R,Zhang S,et al. Cancer statistics in Chinese older people,2022: current burden,time trends,and comparisons with the US,Japan,and the Republic of Korea[J]. Sci China Life Sci,2023,66(5): 1079-1091.4a3帝国网站管理系统
[7] 程明,颜上程,彭巍,等. 日本早期胃癌治疗现状及发展趋势[J]. 中国实用外科杂志,2022,42(10):1173-1179.4a3帝国网站管理系统
[8] Zhang X,Liu B,Wang R,et al. Current status of neoadjuvant immunotherapy for the treatment of gastric cancer[J]. Clin Transl Oncol,2024,26(9): 2097-2108. 4a3帝国网站管理系统
[9] 周志军,徐岩,金芳,等. 晚期胃癌免疫治疗研究进展[J]. 中国实用外科杂志,2022,42(12): 1421-1427.4a3帝国网站管理系统
[10] 李国新. 胃癌微创外科治疗的难点及发展趋势[J]. 中国实用外科杂志,2023,43(1): 77-82.4a3帝国网站管理系统
[11] 葛晗,张殿彩,徐泽宽. 第6版日本《胃癌治疗指南》更新要点解读[J]. 中国实用外科杂志,2022,42(1): 35-40.4a3帝国网站管理系统
[12] Li ZY,Tang L,Li ZM,et al. Four-point computed tomography scores for evaluation of occult peritoneal metastasis in patients with gastric cancer: A region-to-region comparison with staging laparoscopy[J]. Ann Surg Oncol,2020,27(4):1103-1109. 4a3帝国网站管理系统
[13] Ajani JA,D'Amico TA,Bentrem DJ,et al. Gastric cancer,version 2.2022,NCCN clinical practice guidelines in oncology[J]. J Natl Compr Canc Netw,2022,20(2):167-192.4a3帝国网站管理系统
[14] Lordick F,Carneiro F,Cascinu S,et al. ESMO Clinical Practice Guideline for diagnosis,treatment and follow-up[J]. Ann Oncol,2022,33(10):1005-1020.4a3帝国网站管理系统
[15] Wang FH,Zhang,XT Tang L,et al. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer,2023[J]. Cancer Commun (Lond),2023,44(1):127-172.4a3帝国网站管理系统
[16] Cunningham D,Allum WH,Stenning SP,et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer[J]. N Engl J Med,2006,355(1):11-20.4a3帝国网站管理系统
[17] Ychou M,Boige V,Pignon JP,et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: An FNCLCC and FFCD multicenter phase III trial[J]. J Clin Oncol,2011,29(13): 1715-1721.4a3帝国网站管理系统
[18] Al-Batran SE,Hofheinz RD,Pauligk C,et al. Histopathological regression after neoadjuvant docetaxel,oxaliplatin,fluorouracil,and leucovorin versus epirubicin,cisplatin,and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): Results from the phase 2 part of a multicentre,open-label,randomised phase 2/3 trial[J]. Lancet Oncol,2016,17(12): 1697-1708. 4a3帝国网站管理系统
[19] Zhang X,Liang H,Li Z,et al. Perioperative or postoperative adjuvant oxaliplatin with S-1 versus adjuvant oxaliplatin with capecitabine in patients with locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D2 gastrectomy (RESOLVE): an open-label,superiority and non-inferiority,phase 3 randomised controlled trial[J]. Lancet Oncol,2021,22(8): 1081-1092.4a3帝国网站管理系统
[20] Kang YK,Yook JH,Park YK,et al. PRODIGY: A phase Ⅲ study of neoadjuvant docetaxel,oxaliplatin,and S-1 plus surgery and adjuvant S-1 versus surgery and adjuvant S-1 for resectable advanced gastric cancer[J]. J Clin Oncol,2021,39(26): 2903-2913.4a3帝国网站管理系统
[21] Janjigian YY,Ajani JA,Moehler M,et al. First-line nivolumab plus chemotherapy for advanced gastric,gastroesophageal junction,and esophageal adenocarcinoma: 3-year follow-up of the phase Ⅲ CheckMate 649 trial[J]. J Clin Oncol,2024,42(17):2012-2020.4a3帝国网站管理系统
[22] Xu J,Jian H,Pan Y,et al. Sintilimab plus chemotherapy for unresectable gastric or gastroesophageal junction cancer the Orient-16 randomized clinical trial[J]. JAMA,2023,330(21): 2064-2074. 4a3帝国网站管理系统
[23] Rha SY,Oh DY,Yañez P,et al. pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): A multicentre,randomised,double-blind,phase 3 trial[J]. Lancet Oncol,2023,24(11):1181-1195.4a3帝国网站管理系统
[24] Tang Z,Wang Y,Liu D,et al. The Neo-PLANET phase Ⅱ trial of neoadjuvant Camrelizumab plus concurrent chemoradiotherapy in locally advanced adenocarcinoma of stomach or gastroesophageal junction[J]. Nat Commun,2022,13(1):6807.4a3帝国网站管理系统
[25] Wei J,Lu X,Liu Q,et al. Neoadjuvant sintilimab in combination with concurrent chemoradiotherapy for locally advanced gastric or gastroesophageal junction adenocarcinoma: a single-arm phase 2 trial[J]. Nat Commun,2023,14(1):4904.4a3帝国网站管理系统
[26] Bang YJ,Van Cutsem E,Feyereislova A,et al. TRAStuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3,open-label,randomised controlled trial[J]. Lancet,2010,376(9742):687-697.4a3帝国网站管理系统
[27] Gong J,Liu T,Fan Q,et al. Optimal regimen of tRAStuzumab in combination with oxaliplatin/capecitabine in first-line treatment of HER2-positive advanced gastric cancer (CGOG1001): A multicenter,phase Ⅱ trial [J]. BMC Cancer,2016,16:68.4a3帝国网站管理系统
[28] Kagawa S,Muraoka A,Kambara T,et al. A multi-institution phase Ⅱ study of docetaxel and S-1 in combination with trastuzumab for HER2-positive advanced gastric cancer (DASH study) [J]. Cancer Chemother Pharmacol,2018,81(2): 387-392.4a3帝国网站管理系统
[29] Hofheinz RD,Hegewisch-Becker S,Kunzmann V,et al. Trastuzumab in combination with 5-fluorouracil,leucovorin,oxaliplatin and docetaxel as perioperative treatment for patients with human epidermal growth factor receptor 2-positive locally advanced esophagogastric adenocarcinoma: A phase Ⅱ trial of the Arbeitsgemeinschaft Internistische Onkologie Gastric Cancer Study Group[J]. Int J Cancer,149(6),1322-1331. 4a3帝国网站管理系统
[30] Tokunaga M,Machida N,Mizusawa J,et al. Early endpoints of a randomized phase Ⅱ trial of preoperative chemotherapy with S-1/CDDP with or without trastuzumab followed by surgery for HER2-positive resectable gastric or esophagogastric junction adenocarcinoma with extensive lymph node metastasis: Japan Clinical Oncology Group study JCOG1301C (Trigger Study) [J]. Gastric Cancer,27(3):580-589.4a3帝国网站管理系统
[31] Hofheinz RD,Merx K,Haag GM,et al. FLOT versus FLOT/trastuzumab/Pertuzumab perioperative therapy of human epidermal growth factor receptor 2-positive resectable esophagogastric adenocarcinoma: A randomized phase Ⅱ trial of the AIO EGA study group[J]. J Clin Oncol,2022,40: 3750-3761. 4a3帝国网站管理系统
[32] Zheng Y,Yang X,Yan C,et al. Effect of apatinib plus neoadjuvant chemotherapy followed by resection on pathologic response in patients with locally advanced gastric adenocarcinoma: A single-arm,open-label,phase Ⅱ trial[J]. Eur J Cancer,2020,130:12-19.4a3帝国网站管理系统
[33] Tang Z,Wang Y,Yu Y,et al. Neoadjuvant apatinib combined with oxaliplatin and capecitabine in patients with locally advanced adenocarcinoma of stomach or gastroesophageal junction: A single-arm,open-label,phase 2 trial[J]. BMC Med,2022,20(1):107.4a3帝国网站管理系统
[34] Lin JX,Tang YH,Zheng HL,et al. Neoadjuvant Camrelizumab and apatinib combined with chemotherapy versus chemotherapy alone for locally advanced gastric cancer: A multicenter randomized phase 2 trial[J]. Nat Commun,2024,15(1):41.4a3帝国网站管理系统
[35] Inamoto R,Takahashi N,Yamada Y. Claudin18.2 in advanced gastric cancer[J]. Cancers,2023,15(24):5742.4a3帝国网站管理系统
[36] Zhang ZX,Gu XZ,Yin WB,et al. Randomized clinical trial on the combination of preoperative irradiation and surgery in the treatment of adenocarcinoma of gastric cardia (AGC)--report on 370 patients[J]. Int J Radiat Oncol Biol Phys,1998,42(5):929-934.4a3帝国网站管理系统
[37] van Hagen P,Hulshof MC,van Lanschot JJ,et al. Preoperative chemoradiotherapy for esophageal or junctional cancer[J]. N Engl J Med,2012,366(22):2074-2084.4a3帝国网站管理系统
[38] Stahl M,Walz MK,Stuschke M,et al. Phase Ⅲ comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction[J]. J Clin Oncol,2009,27(6):851-856.4a3帝国网站管理系统
[39] Shu Y,Zhang W,Hou Q,et al. Prognostic significance of frequent CLDN18-ARHGAP26/6 fusion in gastric signet-ring cell cancer[J]. Nat Commun,2018,9(1): 2447. 4a3帝国网站管理系统
[40] Smyth EC,Fassan,M,Cunningham D,et al. Effect of pathologic tumor response and nodal status on survival in the medical research council adjuvant gastric infusional chemotherapy trial[J]. J Clin Oncol,2016,34(23):2721-2727.4a3帝国网站管理系统
[41] Schmidt T,Sicic L,Blank S,et al. Prognostic value of histopathological regression in 850 neoadjuvantly treated oesophagogastric adenocarcinomas[J]. Br J Cancer,2014,110(7):1712-1720.4a3帝国网站管理系统
[42] Eisenhauer EA,Therasse P,Bogaerts J,et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) [J]. Eur J Cancer,2009,45(2):228-247.4a3帝国网站管理系统
[43] Park SR,Lee JS,Kim CG,et al. Endoscopic ultrasound and computed tomography in restaging and predicting prognosis after neoadjuvant chemotherapy in patients with locally advanced gastric cancer[J]. Cancer,2008,112(11):2368-2376.4a3帝国网站管理系统
[44] Bozkaya Y,Özdemir NY,Sezer S,et al. Is serum survivin expression a predictive biomarker in locally advanced gastric cancer patients treated with neoadjuvant chemotherapy? [J]. Cancer Biomark,2018,22(1):143-149.4a3帝国网站管理系统
[45] Chiari D,Orsenigo E,Guarneri G,et al. Effect of neoadjuvant chemotherapy on HER-2 expression in surgically treated gastric and oesophagogastric junction carcinoma: A multicentre Italian study[J]. Updates Surg,2017,69(1):35-43.4a3帝国网站管理系统
[46] 胡建昆,张维汉,陈心足. 从中国CLASS-03a研究的开展看腹腔镜手术联合新辅助化疗的胃癌治疗[J]. 中华胃肠外科杂志,2018,21(2): 138-142.4a3帝国网站管理系统
[47] Li Z,Shan F,Ying X,et al. Assessment of laparoscopic distal gastrectomy after neoadjuvant chemotherapy for locally advanced gastric cancer: A randomized clinical trial[J]. JAMA Surg,2019,154(12):1093-1101.4a3帝国网站管理系统
[48] van der Wielen N,Straatman J,Daams F,et al. Open versus minimally invasive total gastrectomy after neoadjuvant chemotherapy: results of a European randomized trial[J]. Gastric Cancer,2021,24(1):258-271.4a3帝国网站管理系统
[49] Zhong H,Liu X,Tian Y,et al. Comparison of short- and long-term outcomes between laparoscopic and open gastrectomy for locally advanced gastric cancer following neoadjuvant chemotherapy: A propensity score matching analysis[J]. Surg Endosc,2023,37(8):5902-5915.4a3帝国网站管理系统
[50] 俞鹏飞,程向东. 胃癌新辅助治疗后行腹腔镜手术争议与共识[J]. 中国实用外科杂志,2023,43(9): 989-992.4a3帝国网站管理系统